Click here to read the most recent trial update from Adrenas Therapeutics.
ADRENAS’ INVESTIGATIONAL GENE THERAPY MAY OFFER, WITH A ONE-TIME DOSE, THE POTENTIAL FOR PEOPLE WITH CLASSIC CAH TO NATURALLY MAKE THEIR OWN CORTISOL AND ALDOSTERONE.
TO LEARN MORE ABOUT OUR ACTIVE CLINICAL TRIAL, VISIT WWW.CAHGENETHERAPY.COM.
Adrenas Therapeutics proudly partners with the CAH community.
Adrenas Therapeutics is a company that was created with a single mission: to work with scientists, physicians, and patients in developing a gene therapy for people affected by CAH. Adrenas’s investigational one-time gene therapy is now in clinical trials, and was recently granted Fast Track Designation by the FDA.
WHAT IS CONGENITAL ADRENAL HYPERPLASIA?
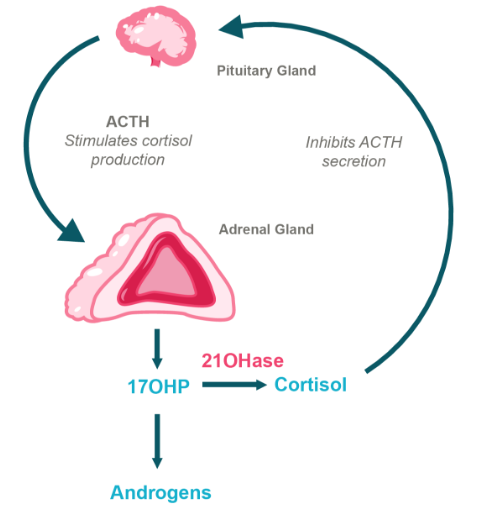
Congenital adrenal hyperplasia (CAH) is a group of genetic disorders that affect the adrenal glands, which sit above each kidney and produce many of the body’s crucial hormones. The most common cause of CAH is a mutation in the gene encoding for 21-hydroxylase, an enzyme essential for making the hormones cortisol and aldosterone which are critical for various physiologic functions. Cortisol is necessary for the body to respond to injury, stress or illness, and aldosterone is required to maintain proper blood pressure and sodium levels.
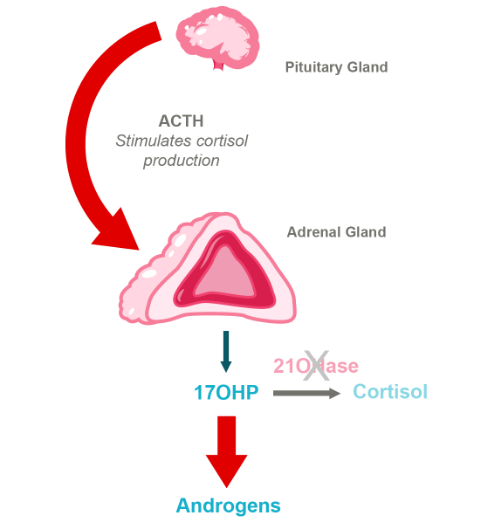
CAH has a severe form known as classic CAH and a milder form known as non-classic CAH. A test for this inherited condition is included on every state’s newborn screening panel. Most individuals with classic CAH cannot produce cortisol, which can lead to an acute, life-threatening condition called adrenal crisis. Patients with salt-wasting CAH (75% of classic CAH) cannot produce sufficient aldosterone, which is important for sodium retention.
Unable to produce cortisol and aldosterone, people with CAH cannot mount the healthy physiological response to stressors, such as illnesses, that allows their heart, lungs, kidneys and other organs to compensate for the stress, which can be life-threatening. These adrenal crises can be particularly dangerous for young children.
Moreover, deficiency of 21-hydroxylase results in an excess of hormones like testosterone and progesterone, also known as androgens. These excess androgens affect many aspects of growth and sexual development in both males and females throughout puberty. Continued dysregulation of androgens often leads to infertility in men and women and a variety of other issues.
Patients with CAH Face a Great Deal of Unmet Need
Treatment of symptoms and risks of CAH are different in children versus in adults, and the main tool currently available for CAH management is the replacement of cortisol produced from synthetic sources.
Lifelong steroid treatment can have significant side effects, including metabolic and bone disease, obesity, psychiatric effects, and effects on the cardiovascular and immune systems. Additionally, these treatments often fail to control excess androgen levels, leaving many symptoms of the disease un-managed. Lastly, steroid supplementation does not recreate the normal daily rhythm of hormone production, which can lead to fatigue and sleep irregularities.
To learn more about CAH, please visit our partners at National Adrenal Diseases Foundation, Adrenal Insufficiency United, the CARES Foundation, the Magic Foundation, and CAH is US.
Normal Hypothalamic Pituitary Adrenal Axis
HPA Axis in CAH Due to 21-Hydroxylase Deficiency
ABOUT GENE THERAPY AND OUR APPROACH
Gene therapy aims to treat genetic conditions by enabling the body to produce a critical protein that is missing, typically with a one-time infusion. Gene therapy does this by using a delivery vehicle to provide a functioning copy of a gene into cells that are missing or do not have a fully functioning copy of that gene.
People with CAH due to 21-hydroxylase deficiency have changes (mutations) in the CYP21A2 gene. The CYP21A2 gene is important for the production of 21-hydroxylase, a critical enzyme necessary for proper function of the adrenal glands. Mutations in CYP21A2 cause 21-hydroxylase to either not be produced or not be fully functional.
Adrenas’ investigational gene therapy, BBP-631, uses a common virus called adeno-associated virus (AAV) as the delivery vehicle for the CYP21A2 gene. You can think of AAV as a “delivery truck,” with the cargo being a functional gene. AAVs used in gene therapy are not associated with any known diseases in people, which is why they are used in gene therapy as the transport vehicles to deliver functioning genes into the body.
Although AAV gene therapy is new to CAH, it’s not new. A number of AAV gene therapies have been approved by the FDA. Gene therapies have also been studied extensively in clinical trials for adults with hemophilia. In total, gene therapies have been used to treat more than 3,500 people around the world, and more patients are being treated with gene therapy every day. There are currently 100+ active AAV gene therapy trials for a variety of diseases. Long-term safety information for both the approved gene therapies and those still in development is being collected continuously. The American Society of Gene and Cell Therapy and has additional information about gene therapies on its website.
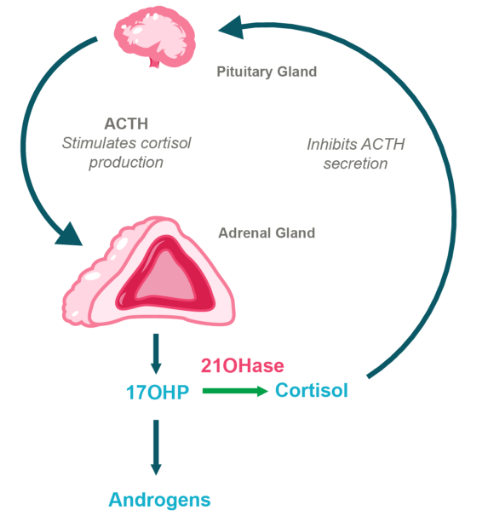
If successful, gene therapy for CAH may restore the body’s hormone and steroid balance by enabling people with CAH to naturally make their own cortisol and aldosterone. It could also allow for people with CAH to eliminate or significantly reduce their daily glucocorticoid or mineralocorticoid doses.
To learn more about the potential of gene therapy for CAH, visit our information and resource center.
How AAV Gene Therapy Works
1
2
3
4
5
6
7
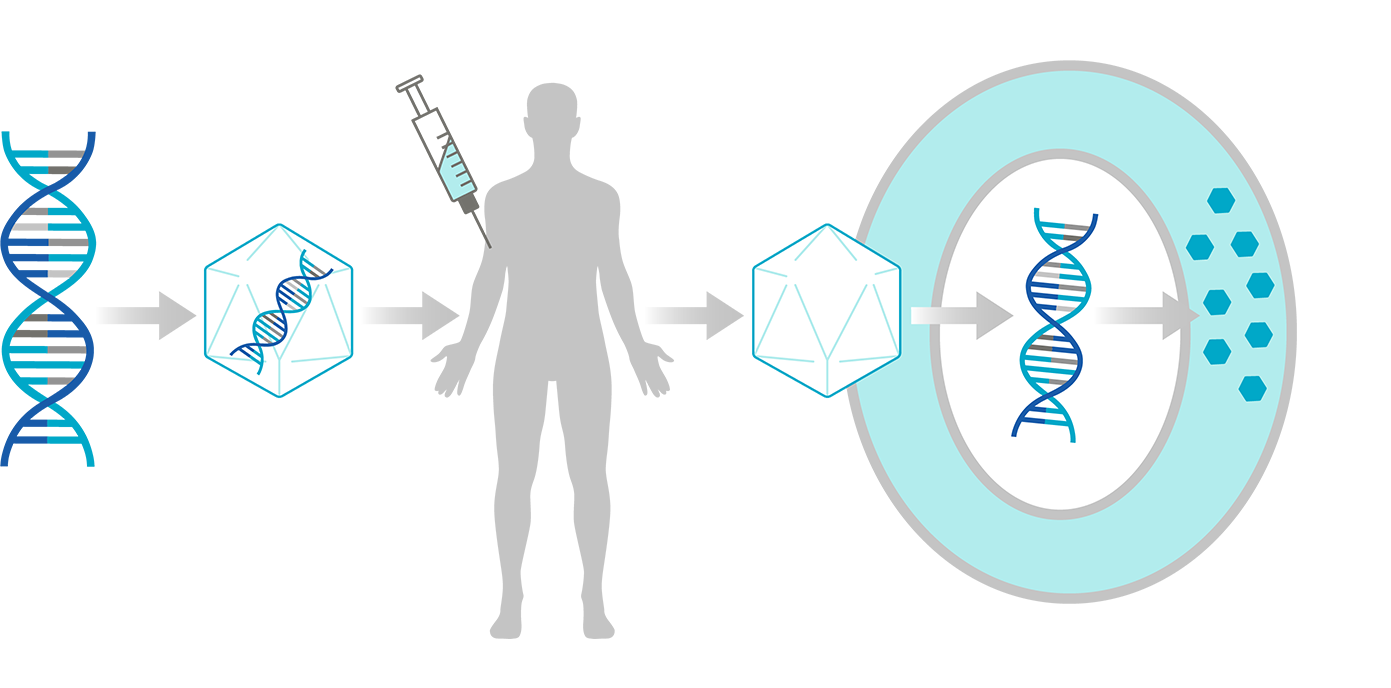
1
DNA
(GENE)
2
GENE ENCAPSULATED IN AAV
3
GENE THERAPY
4
AAV RELEASES GENE INTO CELL
5
TARGET CELL
6
GENE EXPRESSES PROTEINS
7
PROTEIN
HPA Axis in CAH Due to 21OHD
BBP-631 is designed to restore 21-hydroxylase
We have tested our CAH gene therapy in a mouse model with a mutation in the 21 hydroxylase gene. When untreated, the affected mice have poor weight gain and have severely elevated progesterone levels. Mice treated with gene therapy showed increased body weight and reduced progesterone levels, similar to normal mice.
Furthermore, we have performed testing in additional animal models, such as non-human primates, and have observed no significant safety findings in over 20 treated animals. Importantly, these animals show stable gene expression at least six months after treatment, indicating the potential for a durable treatment effect. We have recently presented the data from this experiment at the 2019 European Society of Gene and Cell Therapy conference. Poster available for more information.
ABOUT US
Adrenas Therapeutics is a company that was created with a single mission: to work with scientists, physicians, and patients in developing a gene therapy for people affected by CAH. Adrenas is a subsidiary of BridgeBio, a public company dedicated to finding, developing, and delivering breakthrough medicines for genetic diseases to patients as quickly and safely as possible.
LEADERSHIP
CLINICAL ADVISORY BOARD
RA
Richard Auchus, MD, PhD
The James A. Shayman and Andrea S. Kevrick Professor of Translational Medicine, Professor of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology & Diabetes (MEND) at the University of Michigan
ES
Ellen Seely, MD
Professor, Harvard Medical School and Director Clinical Research, Endocrinology, Diabetes and Hypertension Division at the Brigham and Women’s Hospital
PARENT COMPANY
Adrenas Therapeutics is a member of the BridgeBio family.
BridgeBio is a team of experienced drug discoverers, developers and innovators working to create life-altering medicines that target well-characterized genetic diseases at their source. BridgeBio was founded in 2015 to identify and advance transformative medicines to treat patients who suffer from Mendelian diseases, which are diseases that arise from defects in a single gene, and cancers with clear genetic drivers. BridgeBio’s pipeline of over 20 development programs includes product candidates ranging from early discovery to late-stage development.
NEWS
Trial Update from Adrenas Therapeutics
03.22.2023
First Participant Dosed
01.27.2022
Gene Therapy for CAH Clinical Trial Posting
03.05.2021
CONTACT
1001 William Moore Drive, Suite 100
Raleigh, NC 27607
For any patient-related inquiries, please email [email protected]. For additional information about our upcoming clinical trial, please visit CAHGeneTherapy.com or contact [email protected].